TUESDAY, June 14, 2022 (HealthDay Information)
The primary tablet to regard adults with serious alopecia was once accepted by means of the U.S. Meals and Drug Management on Monday.
Olumiant (baricitinib) is the primary FDA-approved alopecia treatment that treats all of the frame moderately than a particular spot, the company stated in a information free up pronouncing the approval.
“Get entry to to secure and efficient remedy choices is a very powerful for the numerous selection of American citizens suffering from serious alopecia,” Dr. Kendall Marcus, director of the Department of Dermatology and Dentistry within the FDA’s Heart for Drug Analysis and Analysis on the FDA, stated within the information free up. “Lately’s approval will lend a hand satisfy an important unmet want for sufferers with serious alopecia areata.”
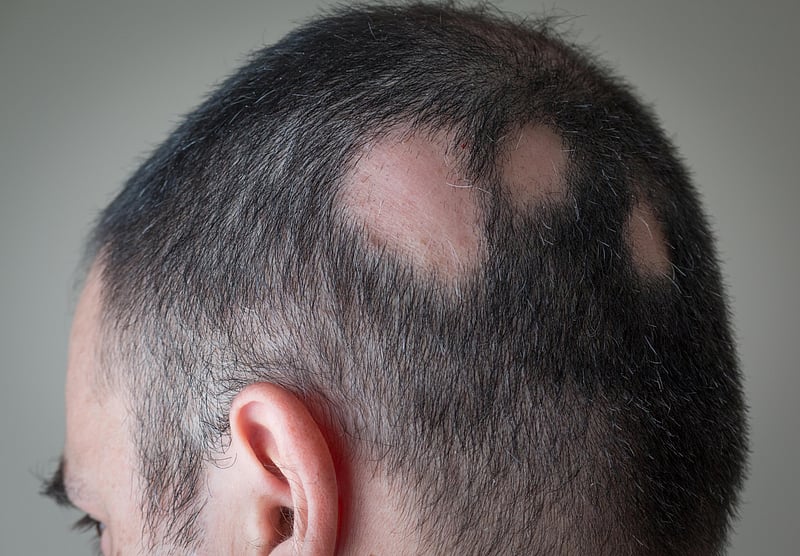
Alopecia areata is an autoimmune dysfunction through which the frame assaults its personal hair follicles, inflicting hair to fall out, steadily in clumps. It impacts greater than 300,000 other folks in the US each and every 12 months, consistent with the FDA.
A kind of is actress Jada Pinkett Smith, who first published her struggles with hair loss in 2018.
For most of the people, the illness comes to one or a couple of small bald patches at the head. However the ones with serious circumstances might understand small bald spots on their heads at some point, after which they not have any hair on their our bodies 3 months, and even 3 weeks, later.
Take the case of Christian Daniels. The 27-year-old knowledge heart technician from Peoria, In poor health., stated his hair began falling out when he was once 25. Inside a month, all of his frame hair was once long past.
Even his imaginative and prescient was once affected: With out eyelashes, mud would get into his eyes and worsen them such a lot he started striking Vaseline on his eyelids.
The pandemic was once a “blessing in hide” as a result of he may do business from home.
“I felt like my lifestyles were placed on dangle,” he instructed The New York Instances. “I felt like the one factor that mattered was once tips on how to get my hair again.”
Now, after being a part of an ordeal of the drug that brought about the FDA approval, “It is virtually love it [alopecia] by no means came about,” he stated, even if he nonetheless appears in a replicate occasionally and has a flashback to his hairless self.
Olumiant is a Janus kinase (JAK) inhibitor that works by means of interfering with the mobile pathway that triggers irritation. It was once first accepted in 2018 to regard rheumatoid arthritis, the FDA stated.
The company’s approval of the drug from Eli Lilly and Co. is according to two medical trials that integrated 1,200 alopecia sufferers with a minimum of 50% hair loss who took both 2 or 4 milligrams of Olumiant or a placebo each day.
After 36 weeks, charges of sufferers who completed a minimum of 80% hair protection have been 17% and 22% for many who took 2 milligrams of Olumiant and 32% and 35% of those that took 4 milligrams of the drug. That when compared with 3% and 5% of those that took a placebo, consistent with the FDA.
The commonest unwanted effects related to Olumiant integrated: higher breathing tract infections, headache, pimples, prime ldl cholesterol, build up of an enzyme referred to as creatinine phosphokinase, urinary tract an infection, liver enzyme elevations, irritation of hair follicles, fatigue, decrease breathing tract infections, nausea, genital yeast infections, anemia, low selection of sure sorts of white blood cells, belly ache, shingles and weight acquire.

QUESTION
It’s customary to lose 100-150 hairs in line with day.
See Solution
Olumiant must no longer be utilized in mixture with different JAK inhibitors or every other potent immunosuppressants, the FDA warned. The drug carries a boxed caution for critical infections, loss of life, most cancers, primary middle issues and blood clots.
Affected person taking the drug must be carefully monitored for an infection all over and after remedy and must be checked for latent and lively tuberculosis ahead of remedy, the FDA suggested.
With the FDA approval will come insurance policy for those dear medication, that have a listing worth of just about $2,500 a month, The New York Instances reported. Two different corporations, Pfizer and Live performance Prescribed drugs, are shut at the back of Lilly with identical medication which can be already in the marketplace for the remedy of rheumatoid arthritis and different autoimmune sicknesses.
Additional information
There may be extra on alopecia on the U.S. Nationwide Library of Drugs.
SOURCES: U.S. Meals and Drug Management, information free up, June 13, 2022
Through Robin Foster and Robert Preidt HealthDay Newshounds

Copyright © 2021 HealthDay. All rights reserved.

