TUESDAY, Might 3, 2022 (HealthDay Information)
The U.S. Meals and Drug Management is caution American citizens to be careful for phony at-home, over the counter COVID-19 checks that glance so much like the actual issues.
The counterfeit check kits would possibly put you susceptible to unknowingly spreading the illness or now not in search of suitable clinical remedy, the company cautions.
The phonies “are made to seem like permitted checks so the customers will suppose they’re the actual, FDA-authorized check,” the FDA stated in a commentary concerning the fakes. “The FDA is anxious concerning the possibility of false effects when other people use those unauthorized checks.”
For those who get a false studying that you simply do not need the coronavirus, you want to inadvertently infect others at domestic, at paintings or in clinical and long-term care amenities. Additionally, it’s possible you’ll now not search or may discontinue remedy for COVID-19, the company defined.
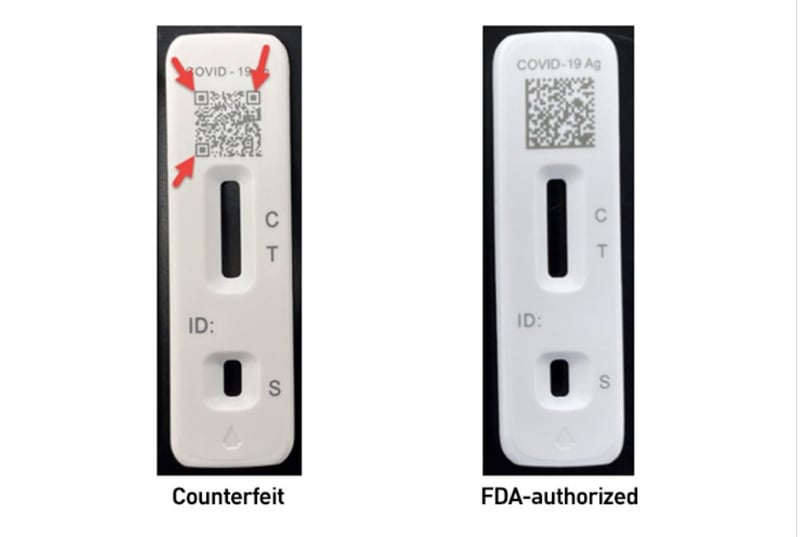
Two fakes the FDA is aware of of include counterfeit Flowflex COVID-19 check kits and iHealth Antigen Speedy Check Kits — you’ll in finding extra main points on easy methods to spot the fakes on the FDA commentary. The bundle and elements of the Flowflex imitation may simply misinform customers searching for the permitted Flowflex check.
Sure pink flags would possibly can help you spot the counterfeits, in keeping with the FDA. They come with:
- Deficient print high quality of pictures or textual content at the outdoor field label or within the directions to be used integrated within the field.
- Lacking data at the outdoor field label for the product, such because the lot quantity, expiration date or barcode or QR codes.
- Grammatical or spelling mistakes in product labeling.
- Equipment elements that don’t fit the content material description. As an example, lacking directions to be used, lacking or unfilled elements, other choice of elements than indexed.
- The tradename for product published on part or field labels vary from the permitted labeling discovered at the FDA site.
- The field label or published directions to be used glance other from the permitted labeling discovered at the FDA site.
The FDA has a listing of permitted at-home OTC COVID-19 checks. It isn’t acutely aware of any counterfeit checks allotted through federal govt check distribution techniques.
What will have to you do if in case you have one?
For those who suspect you’ve got a counterfeit check, don’t use it. Touch the distributor or retailer the place you purchased it to inform them that you’ve got a counterfeit check, and in addition tell the producer of the permitted check, the company stated.
The producer would possibly ask for additional info comparable to pictures of the packaging to additional examine the problem. After offering any asked data to the distributor and/or producer, observe the producer’s directions for returning or taking away the check.
Communicate for your well being care supplier if you happen to suppose you had been examined with a counterfeit check and you’ve got issues about your effects, the FDA urged.
For those who suppose you had an issue with a COVID-19 check, you’ll record it throughout the FDA’s MedWatch Voluntary Reporting Shape.
Additional information
The U.S. Facilities for Illness Keep watch over and Prevention outlines what you wish to have to learn about COVID-19 checking out.
SOURCE: U.S. Meals and Drug Management, information unencumber, April 28, 2022
By way of Robert Preidt HealthDay Reporter

Copyright © 2021 HealthDay. All rights reserved.
